Berlanga: Political decision makers can save people from amputation
Seven years ago, one of the greatest findings of Cuban science was announced. At the laboratories of the Center for Genetic Engineering and Biotechnology (CIGB) in Havana, a formula was developed that has benefited more than 148,000 diabetes patients worldwide.
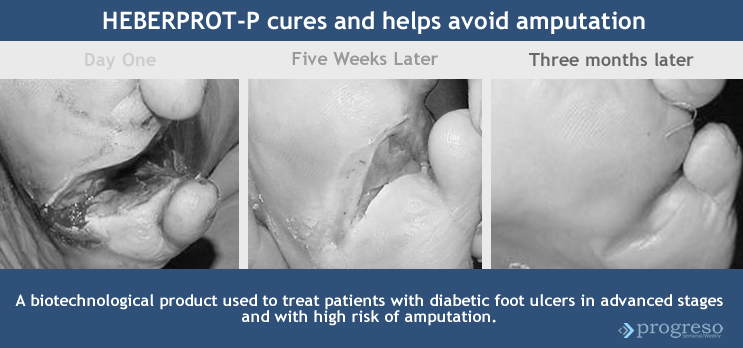
Heberprot-P is its name. It is a medication that provides a therapy of replacement, effective and unique, aimed at promoting healing in diabetic-foot ulcers and therefore to reduce the risk of amputation.
The visible advances in the application of this product to more than 28,000 Cuban diabetes patients has enabled its acceptance in countries like Venezuela, Argentina and Turkey and its expansion in Ecuador and Algeria. It is registered in 20-some countries and is expected to be registered in 20 others by 2015.
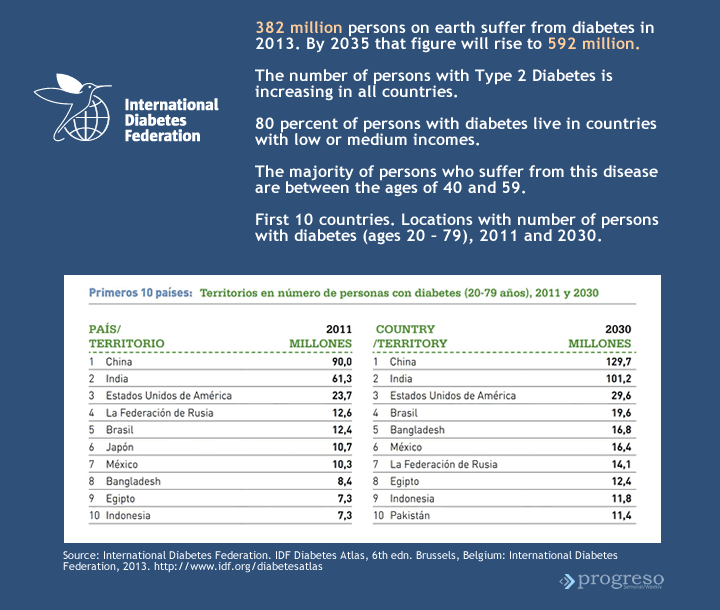
Heberprot-P has made its way in a worldwide context that demands the use of strategies to treat a disease that will affect 592 million people by 2035. The United States is one of the countries (behind China and India) with the largest number of people affected by diabetes mellitus and its complications.
That is why the interest in the Cuban experience has grown among doctors, academicians and researchers in the United States. But the complex political relations between the two countries make the use of this product in the U.S. impossible.
Nevertheless, this year, for the first time, Jorge Berlanga, the scientist who is considered to be the father of Heberprot-P was able to present the results of his findings in Washington and Los Angeles, a step that many consider hopeful.
Progreso Weekly wanted to know his impressions about that experience and about the upcoming international conference on diabetes control that will be held in Cuba next December.
Lisandra Díaz Padrón: Please give us a brief history of Heberprot-P.

Jorge Berlanga: In 1984, before the CIGB was inaugurated, the man who cloned and expressed the protein-coding genes for the Epidermal Growth Factor (EGF) was the CIGB’s current director, Luis Herrera. By 1987, Cuba had purified EGF as a mature protein. We were the second country to do so, after the United States. The first line to be developed was topical formulation. Treating a wound, we instinctively apply medication on it, whereas the mechanism of wound healing goes from below the wound to the surface.
In 1991, we began to work on aspects of the pharmacology related to the EGF. In 1994, we started a parallel line of research where we injected the EGF. To be honest, I was never completely satisfied with the results of its topical application. The experiments told us that that was not the adequate way to apply it and that led us to develop the theory that by injecting EGF on the edges and bottom of the ulcer we could rescue and regenerate the tissues.
The first clinical trials began in 2001. We could do them because here, at the Center, we had large quantities of EGF obtained through a recombinant process, with a high degree of purity, identical to the one we humans have. The main message sent by all those studies was that the pharmacology of the topical administration had nothing to do with the injectable administration.
At first, we had 15 patients. By late 2002 we had 29. Of these, 17 finished the treatment and saved their limbs. Let us say that it was the launching pad for the Center, for the group that its director, Dr. Luis Herrera, has articulated and led to develop the formula we now have, the world’s only growth factor manufactured for injectable use.
LD: You say that you had never participated in the most important event to bring together U.S. professionals who treat diabetic-foot patients. How did you manage to participate this year?
JB: David Armstrong, a celebrity on the issue of diabetic-foot ulcers, sponsors events of this type, from a clinical point of view, and he sent us an invitation. He participated in the Heberprot-P conference held in Varadero in 2012. Also, I knew him from the World Conference of Wound Healing Societies in Toronto in 2008.
When he visited us, he had an opportunity to visit the Angiopathy Wing of El Rincón Hospital, where he became acquainted with the product. Besides, he read all the literature and in fact we have collaborated in publications. He has familiarized himself with Heberprot-P and has enabled our team to communicate with other members of the scientific and medical communities in the United States.
LD: What was the experience like?
JB: My lecture followed that of Professor Andrew Bolton’s, president of the European Association for the Study of Diabetes. That event brings together celebrities from the U.S. and other countries every year in March. Through that exchange, the attendees catch up with the surgical treatments, alternative and pharmacological treatments.
Going there was something extraordinary. Undoubtedly, a great opportunity to introduce the product and being able to tell about the benefits we have brought to any human being who is or was on a hospital bed.
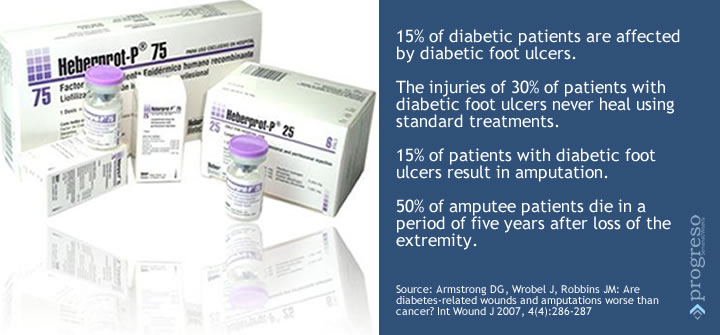
In the United States, between 70,000 and 80,000 people undergo amputation of diabetic foot every year. The care of the wound is expensive, the expenses include surgery, anesthesia, medicine, hospital stay. How much better then to undergo a therapy that within days begins to show results in the most complex lesions?
LD: So, this opportunity can lead to other roads?
JB: In truth, I never imagined that the medicine and my presentation could be so well received. Many people were connected to the network of the Pan-American Health Organization. I believe that all countries in Latin America were aware of the product, too.
By this, I don’t mean that this signifies a victory. We have to see it as a healthy satisfaction, the exaltation of the values and principles of José Martí, because Homeland is Humanity. We don’t believe that this opportunity is reason enough to be boastful. It is simply the ability to share an achievement of Cuba’s revolutionary science with the American people. That simple.
Heberprot-P is at their disposal. So, those who write the laws, who make political decisions, have in their hands the power to help their compatriots avoid amputation. We are willing to provide it to whomever needs it, without boasting or preening; we’ll do it at any time, with the greatest humility.
LD: How does an American patient deal with this disease?
JB: They have different alternatives, none of them pharmacological. Their workhorse is a pump that creates a vacuum over the wound. It is an expensive device that is rented to hospitals. The mechanism has a sort of suction cup that adheres to the intact skin, adjacent to the wound, and creates a vacuum.
According to some specialists, the pump stimulates healing. True, it removes the exudate, the pus and the contaminated matter. But closing the wound, that’s something else. They use a lot of bandages and dressings for smaller lesions. But Heberprot-P is an “orphan/niche drug” used for lesions of greater complexity that have no treatment and almost always end up in amputation.
LD: In addition to noncommercialization, what other aspects related to Heberprot-P are frustrated by the U.S. government’s policy against Cuba?
JB: Even the development of human relations. For example, I may be the Cuban researcher with the largest number of friends among U.S. researchers and doctors, and I have realized that they fear to communicate, fear to exchange knowledge, fear to donate, fear to do anything.
The main problem sometimes is not the limitations to acquire a reactive, or a resource or a kit. Sometimes the greatest limitation lies in the inability to debate, to exchange, to hear the advice of a person who has spent years doing what you are just beginning to do, and who can give you the know-how, the tricks of the trade.
For us to visit the United States, we have to do it through an international organization, in the latest instance through the Pan-American Health Organization. That doesn’t mean that if we had applied for a visa Washington would have denied it. We don’t know. In the philosophy and mentality of some people over there, Cuba is neither authorized nor entitled to develop a biotechnological industry.
LD: This year, the third congress on the subject will be held and your projection has changed. You now want to encompass everything related to the integral care of the diabetic patient. Why have you broadened the spectrum?
JB: We cannot limit ourselves to the patient’s foot. There is a two-way relationship between wound and host, so we don’t accomplish anything by treating a wound if we cannot control the host, i.e., the diabetic patient, all the more when the wound is an expression of a complication in the basic disease. That is why our profile has broadened.
LD: Let us hope for good news for Heberprot-P.
JB: Every step we take, small though it may be, improves the medication’s credibility. And it will continue to do so, because we’re not going to stop.
Progreso Semanal/ Weekly authorizes the total or partial reproduction of the articles by our journalists, so long as source and author are identified.